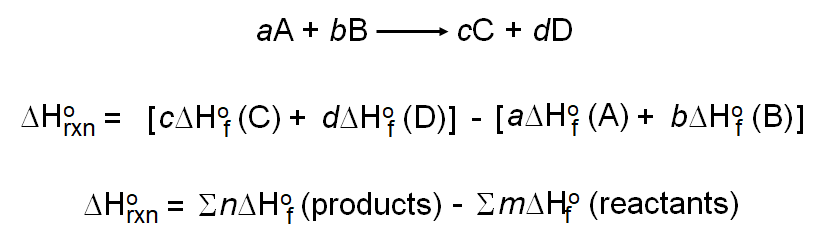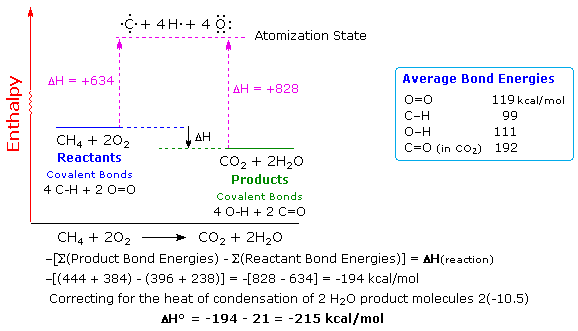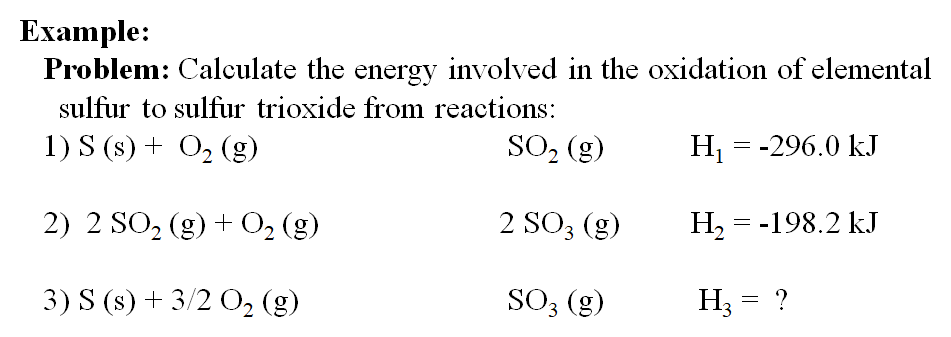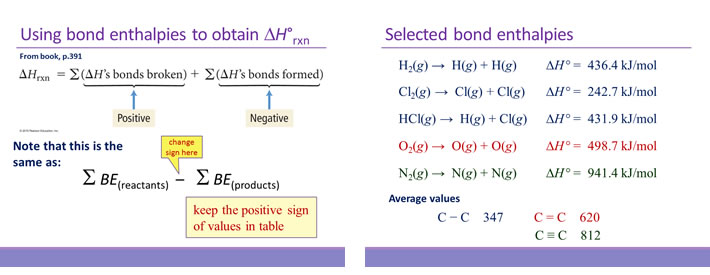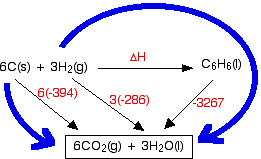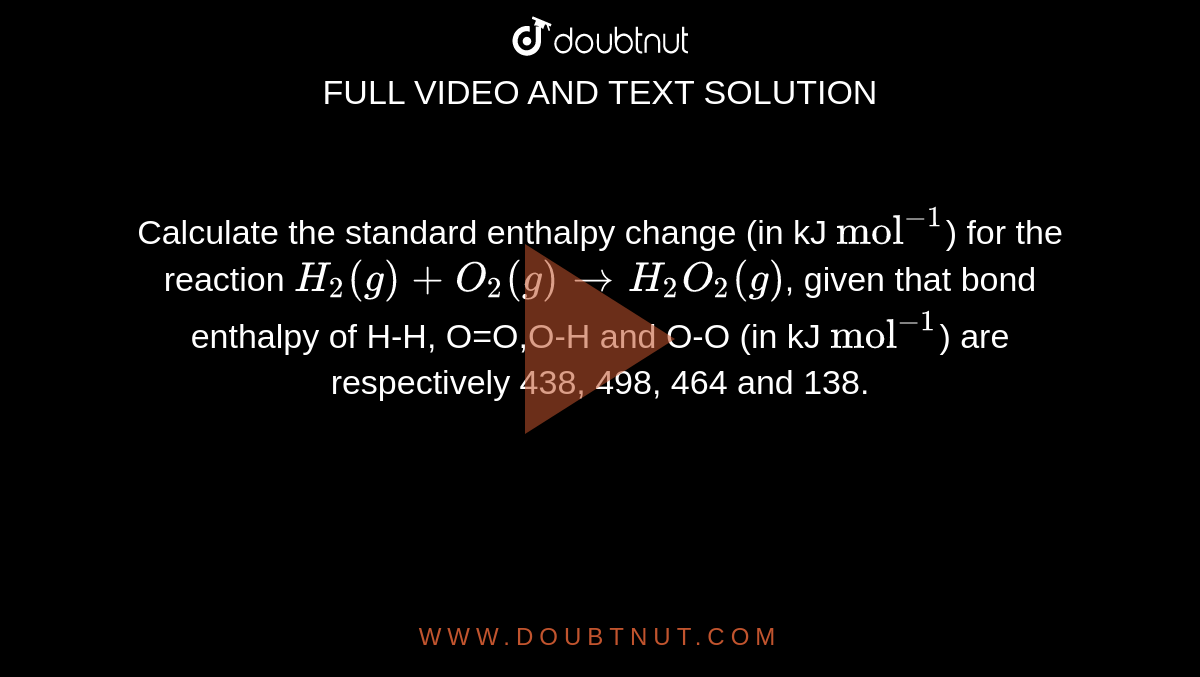
Calculate the standard enthalpy change (in kJ "mol"^(-1)) for the reaction H(2)(g)+O(2)(g)toH(2)O(2)(g), given that bond enthalpy of H-H, O=O,O-H and O-O (in kJ "mol"^(-1)) are respectively 438, 498, 464 and 138.

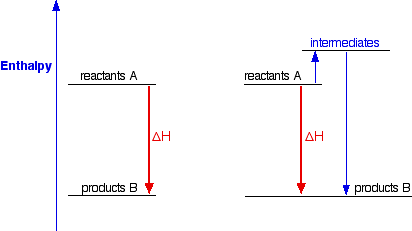
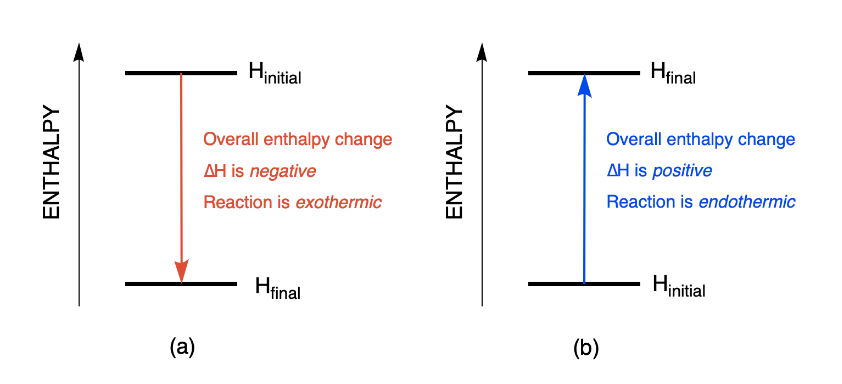
What is the overall enthalpy change DHrxn for the system? -1,300 kJ -300 kJ 300 kJ 1,300 kJ - Brainly.com
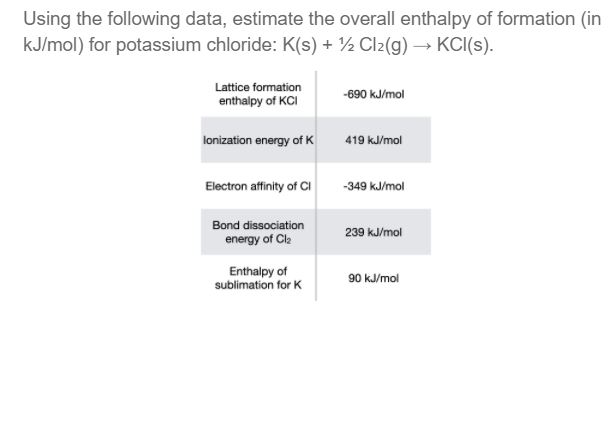
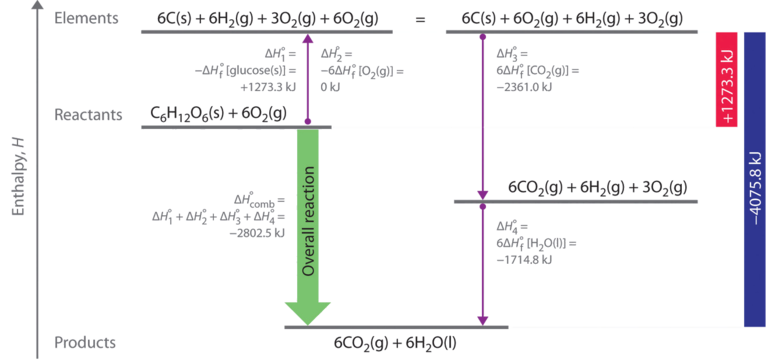
SOLVED: Using the following data, estimate the overall enthalpy of formation (in kJ / mol ) for potassium chloride: K(s)+1 / 2 Cl2( g) →KCl(s) . Lattice formation enthalpy of KCl -690
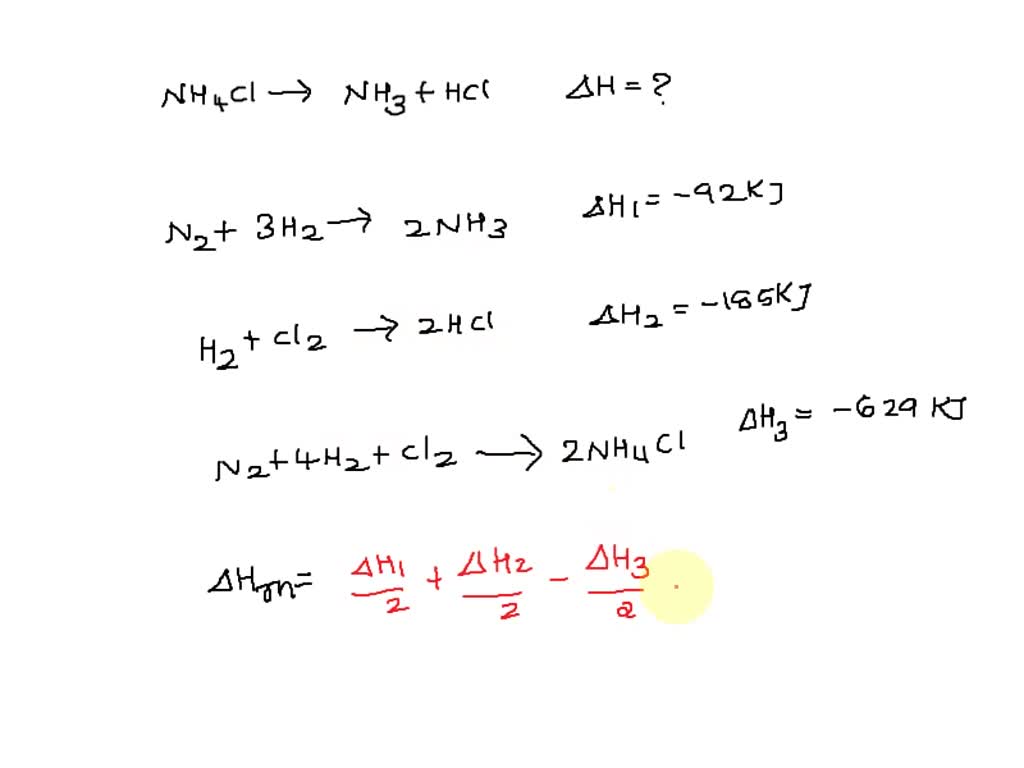
SOLVED: Given the following reactions, what is the overall enthalpy change for the following reaction? NH4Cl(s) —-> NH3(g) + HCl(g) ΔH = ? Use the following reactions to derive the ΔH of